|  This
term is defined in the glossary. This
term is defined in the glossary. |
|
 |
 |
 |
 |
Beaker/Bottle
Calibration & Interpretation
To determine what concentration of insecticide to use when running a
bioassay, it will be necessary to calibrate the beaker or bottle. This
must be done for each insecticide and species of mosquito.
The principle of calibration is the same whether you are preparing to
test the mosquito larvae or adult mosquitoes. However, the procedure itself
differs in one respect: when you test larvae you will calibrate beakers,
while when you test adults you will calibrate bottles.
Data are likely available on the vector species and insecticide to be
tested, but it is desirable for the user to develop their own data. Ideally,
susceptible target are used to establish a baseline. If these are not
available, use mosquitoes from the population to be controlled and use
these data as a benchmark for interpreting future changes in the population.
A range of insecticide concentrations is used.
- Prepare beakers/bottles as explained in the Bottle
bioassay or Larval bioassay. Make
several sets of beakers or bottles with a range of different concentrations.
- Run assays on susceptible mosquitoes/larvae.
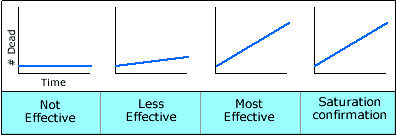
- When the results are plotted as time-mortality data on a graph, you
should see that with increasing concentration the time-mortality line
becomes straighter, steeper and moves toward the Y axis. If you are
in the correct range, the line will reach a point where increasing the
concentration does not change the line's appearance. This is called
the "saturation" point for the insecticide against the insecticide
target site in the mosquitoes. At the saturation point, increasing the
concentration any further will not increase the rate at which the insecticide
penetrates the mosquito and gets to the target site. This is the diagnostic
concentration that you will use durring the bioassays.
- To find the right saturation point, you may have to repeat the assay
using different concentrations (larger or smaller), or smaller increments
between concentrations. For example you may start out with increments
of 100 ug, beginning with 100 ug/beaker and ending at 1000 ug/beaker.
If after these assays you do not see a clear saturation point, you may
need to run more beakers with <100 ug/beaker or >1000 ug/beaker.
On the other hand, if you do see a clear saturation point, you can refine
what that value is by running more beakers at smaller increments near
where you see the break. You can go back and rerun beakers using an
increment of 100 fold concentrations.
CDC typically use insecticide solutions of 100 mg/ml, 10 mg/ml, 1 mg/ml,
and 0.1mg/ml.
Interpreting data:
Like all resistance tests, bioassay data need to be compared to data
from susceptible or base line sources. A resistance threshold for each
insecticide can be determined by drawing a straight line down from the
point at which all of the susceptible mosquitoes have died. Mosquitoes
surviving at or beyond this threshold are interpreted as being less
susceptible to the insecticide. Something in the mosquito is delaying
the insecticide from reaching the target site and acting. In other words,
they have some degree of resistance.
Learn more about Interprating
data and graphs.
|
 |
 |